Innovative stem cell therapy offers heart attack patient a new lease of life
Published on 10/08/2023
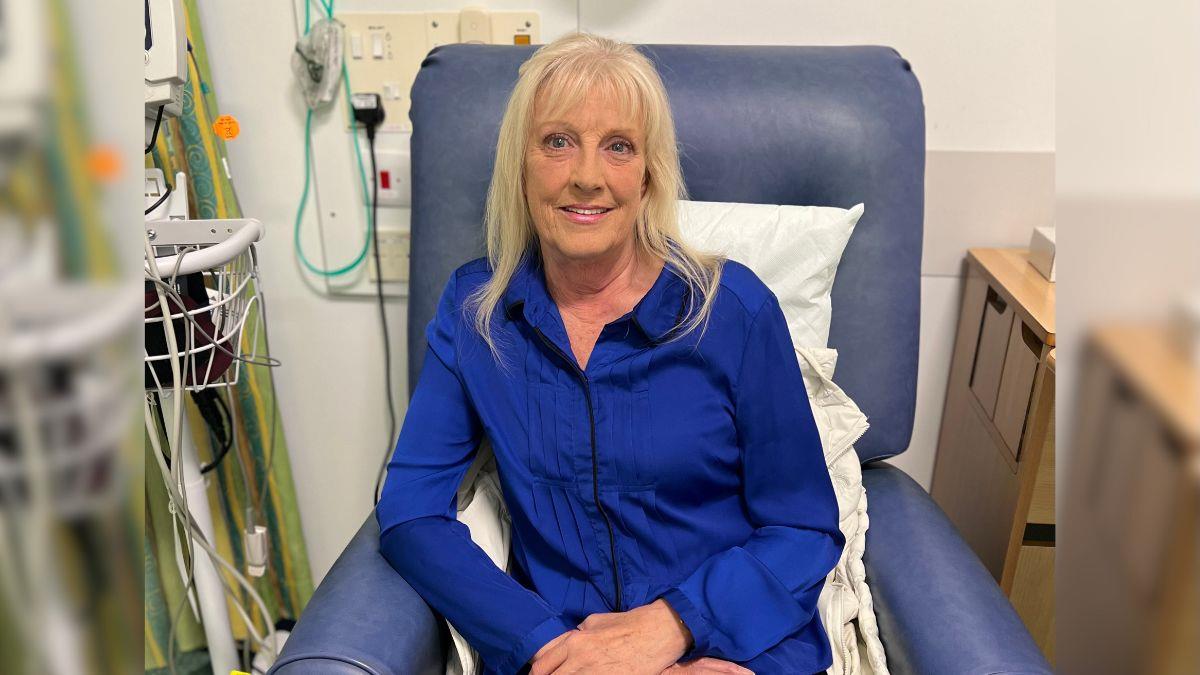
Kim Smith, a 66-year-old heart attack patient, has experienced a life-changing breakthrough after receiving stem cell therapy at the Queen Elizabeth Hospital Birmingham (QEHB), with scans showing almost complete restoration of her heart muscle function just weeks later.
In March this year, Kim had a severe heart attack, which left her heart functioning dangerously low, at just 25%. When discussing her treatment options with her cardiologist, she was offered the opportunity to be part of a research trial, looking at the effectiveness of stem cell therapy in improving the heart muscle function of patients who have had a heart attack.
The therapy, developed by biotechnology company CellProthera, involves the application of a person’s own stem cells directly into their heart, through the femoral artery in the leg. The patient’s heart activity is then monitored for six months, at 1, 3 and 6 months, using echocardiography and magnetic resonance imaging (MRI).
Following a heart attack, around 30% of patients are left with severely damaged and weakened hearts. Damage to the heart muscle can, over time, lead to life-threatening heart failure, as the heart is unable to pump the blood around the body properly. CellProthera’s therapy aims to prevent this heart failure in patients, by regenerating the damaged muscle caused by the heart attack.
Kim Smith is one of four patients recruited at QEHB to the trial and was randomly allocated to the experimental arm of the study, meaning that she received the therapy as treatment following her heart attack. Just two weeks after receiving the therapy, Kim’s heart function had returned to almost normal (55%).
Kim said: “I now feel as though I can actually do what I used to do before. When I had the heart attack, I was worried that I was going to end up being stuck at home all day, but since having the stem cell therapy, psychologically and physically, I just feel so much better.
“I’m so grateful to have had this treatment, and I do hope that the research that they are doing goes forward because I think a lot of people would benefit. That was my reason for doing it in the first place - even if it does nothing for me, it could help someone else.”
So far, approximately 50 patients from the UK and France have been recruited to the trial, known as the EXCELLENT (Expanded Cell Endocardial Transplantation) study. The research is currently in its final phase, with results expected later this year.
Dr Sohail Khan, Consultant Interventional Cardiologist and Lead Investigator at QEHB for the EXCELLENT study, said: “We’ve had great success in recruiting to this trial, and here at the QE we have been one of the biggest recruiters in the UK. What we have seen so far is that actually the stem cells do seem to have a dramatic effect in terms of improving heart muscle function.
“Currently, there are few clinical options available that repair and regenerate heart tissue following a heart attack. As a result, the only option for many patients that have suffered a heart attack and developed advanced heart failure, is a fully invasive heart transplant. This is a very serious procedure for the patient, and very costly for society.
“The development of a cell therapy to regenerate cardiac tissue will be transformative for a considerable number of patients globally. A minimally invasive, cell therapy, that uses a patient’s own stem cells, could also considerably reduce treatment costs.”
Matthieu de Kalbermatten, CEO at CellProthera, said: “Bringing the therapy to market as a minimally invasive therapy is vital to tackle, from the root, the harmful effects of heart attacks and improve quality of so many lives.
“The impressive progress of the CellProthera EXCELLENT trial is a testament to the work of our team and our stakeholders. All of the trial sites in the UK and France are committed to admitting and treating the final patients as quickly as possible. In 2024, we will look to start the phase III trial, where we will be recruiting patients from across Europe, with the aim of potential future market authorisation and bringing this vital treatment to all patients.”


